HDV: Lonafarnib first and only oral agent in development for Hepatitis Delta Virus infection

Lonafarnib is a well-characterized, first-in-class, inhibitor of farnesyl-transferase, an enzyme involved in modification of proteins through a process called prenylation. HDV uses this host cell process inside liver cells to complete a key step in its life cycle. Lonafarnib inhibits the prenylation step of HDV replication inside liver cells and blocks the virus life cycle at the stage of assembly.
Lonafarnib has been granted Orphan Drug Designation by the US FDA and European Medicines Agency (EMA), as well as Fast Track and Breakthrough Therapy Designation by the US FDA and PRIME Designation by the EMA.
Lonafarnib is generally well tolerated. Most commonly reported adverse events to date are diarrhea and nausea. Side effects can be well managed with prophylactic treatment with antidiarrheals and antiemetics. Longest duration of dosing is > 10 years.
Lonafarnib is developed in HDV by Eiger BioPharmaceuticals. Eiger could have 2 HDV regimens (one ‘all-oral’ and one containing PEG-IFNα) approved in US by Q4 2023.
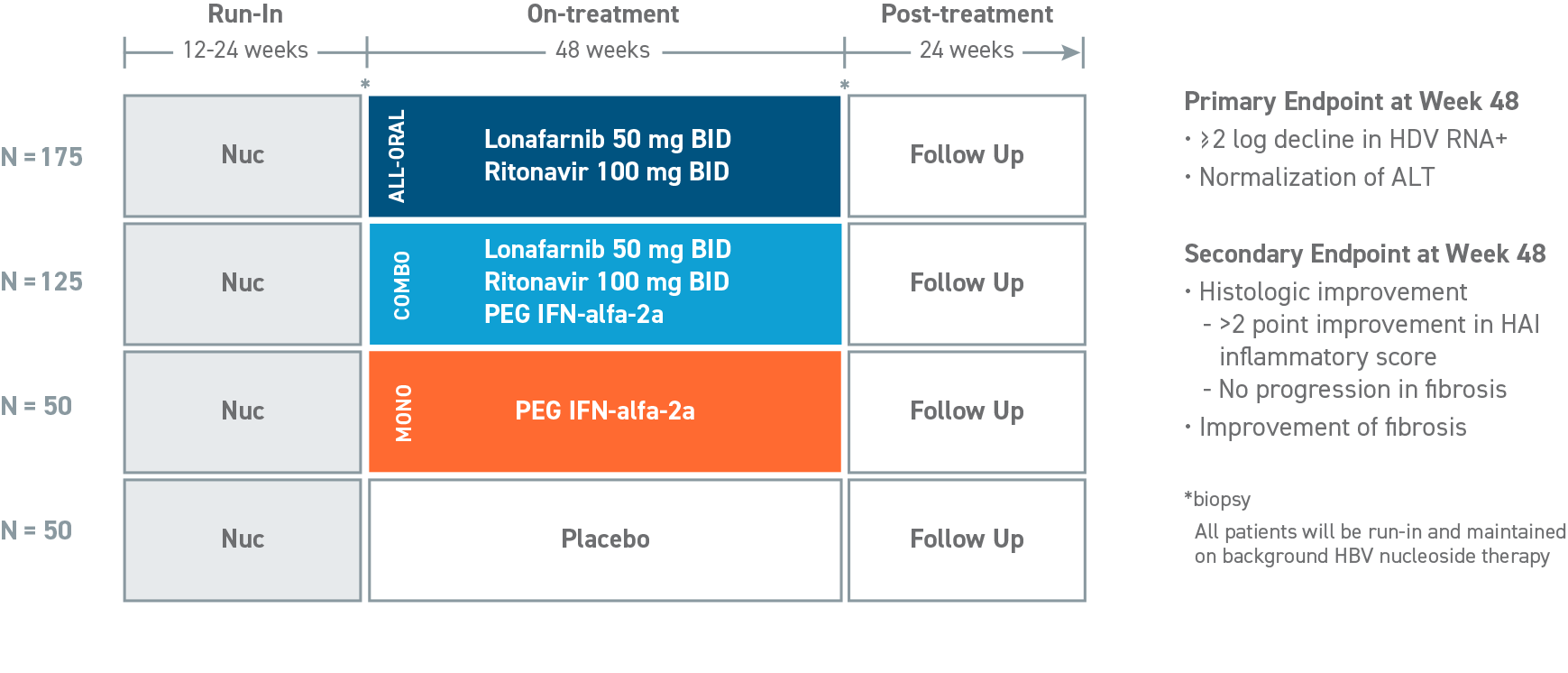
D-LIVR: PHASE 3 GLOBAL STUDY
evaluates an all-oral arm of LNF boosted with RTV and a combination arm of LNF boosted with RTV combined with pegylated interferon-alfa-2a (PEG IFN-alfa-2a), with each arm to be compared with a placebo arm (background HBV nucleos[t]ide only), in HDV-infected patients.