Regulatory news
Analysis of new drug approvals by six major Authorities in 2014-2023

The Centre for Innovation in Regulatory Science (CIRS) published the annual analysis of new active substance (NAS) approvals by six major regulatory agencies:
the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and the Australian Therapeutic Goods Administration (TGA).
The analysis focuses on 2023 as well as looking back at 2014-2023.
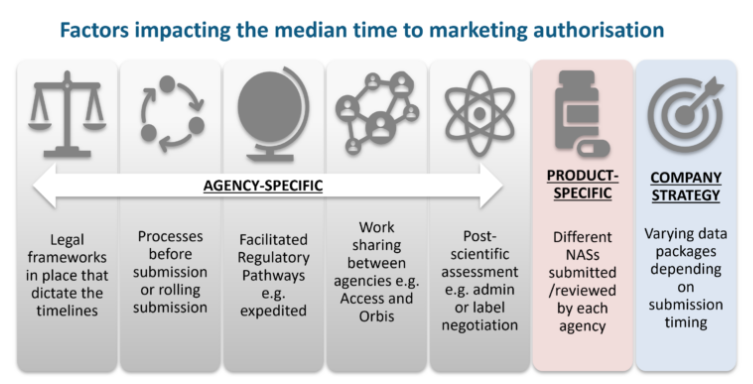
Although median approval times can be a marker of agency performance and the time it takes to make medicines available to patients, other factors must be considered, as illustrated below.
Full Report here
Grazie per il tuo feedback!
